Abstract
Background: Patients with severe hemophilia A experience chronic and acute pain due to joint bleeds and joint degeneration that negatively impacts their health-related quality of life and contributes to the disease burden. Results from the Phase 3 XTEND-1 study demonstrated that once-weekly efanesoctocog alfa prophylaxis was well-tolerated and provided superior bleed protection compared with prior Factor VIII (FVIII) prophylaxis.
Objective: To evaluate the efficacy of once-weekly efanesoctocog alfa prophylaxis (Arm A) on pain in adults and adolescents (≥12 years of age) with severe hemophilia A who were previously on standard of care FVIII prophylaxis.
Methods: XTEND-1 (NCT04161495) was a phase 3, open-label, multicenter trial in previously treated patients ≥12 years of age with severe hemophilia A. Patients received once-weekly prophylactic efanesoctocog alfa, 50 IU/kg for 52 weeks (Arm A) or on-demand efanesoctocog alfa, 50 IU/kg for 26 weeks followed by 26 weeks once-weekly prophylaxis (50 IU/kg; Arm B). Pain was assessed as a secondary endpoint using the Patient-Reported Outcomes Measurement Information System (PROMIS)-Short Form (SF) v1.0 pain intensity 3a first question (item, pain intensity at its worst in the past 7 days), and as exploratory endpoints using the PROMIS-SF v1.0 pain intensity 3a total T-score, PROMIS-SF v1.0 pain interference 6a T-score (in patients ≥ 18 years of age), and EuroQol- 5 Dimension 5-level (EQ-5D-5L) pain/discomfort domain. Pain was further assessed in post-hoc analyses (including psychometric analyses) of PROMIS-SF Pain Intensity 3a and during exit interviews. Pain medication usage was also evaluated. Changes in pain scores from baseline to Week 52 were measured using descriptive statistics. Mixed-effect model with repeated measures was used to estimate the change from baseline to Week 52 in PROMIS Pain intensity 3a, pain at its worst in the past 7 days. Here we report results for patients in Arm A. Results of exit interviews and pain medication usage were evaluated in the overall patients included in the trial (Arms A and B).
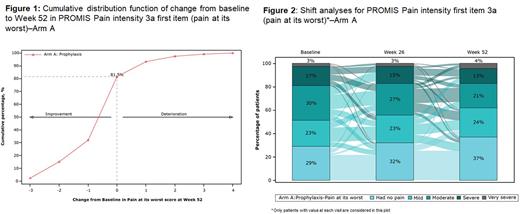
Results: A total of 159 patients (Arm A, N=133; Arm B, N=26) were included in the XTEND-1 trial. At baseline, 53.6% (67/125) patients reported moderate, severe, or very severe pain (at its worst in the past 7 days) on PROMIS-SF Pain intensity 3a scale, while moderate, severe, or extreme pain/discomfort was experienced by 19.7% (24/122) patients on EQ-5D-5L. Prophylactic treatment with efanesoctocog alfa demonstrated statistically significant improvement in PROMIS Pain intensity 3a first item score, with a least square (LS) mean change from baseline to Week 52 of -0.21 (95% confidence interval [CI]: -0.41 to -0.02; P=0.0276). This improvement was also clinically significant since it was within the range of the meaningful within-group change (-0.5 to -0.2) determined using psychometric analyses in the context of the study. These results were supported by improvement in the PROMIS-SF Pain intensity 3a T-score (LS mean change from baseline: -1.94 [95% CI: -3.26 to -0.63]; P=0.0042) and PROMIS-SF Pain Interference 6a T-score (LS mean change from baseline to Week 52: -1.16 [95% CI: -2.45 to 0.14]; P=0.0793). Pain intensity (at its worst) was improved or maintained (change from baseline≤0) for most patients (81.5% [97/119]) at Week 52 (Figure 1). A greater proportion of patients did not experience any pain (37% [44/119]) at Week 52 compared with the baseline (29% [35/119]) in PROMIS pain intensity 3a first item (pain at its worst) scale (Figure 2). At Week 52, 20.9% (24/115) patients reported improvement in pain/discomfort domain from EQ-5D-5L. Consistent with these findings, the overall results from the exit interviews (N=29, including 17 patients in Arm A) reported meaningful improvement in pain in most patients (25/29). The percentage of patients from Arms A and B who did not take any hemophilia-related pain medication in the 14 days prior to visit increased from baseline (73.6%; [n=117]) to Week 52 (81.9% [n=127]), reflecting a trend towards a relative reduction in hemophilia-related pain medication usage at Week 52.
Conclusions: Pain is an important burden in patients with severe hemophilia A. Once-weekly prophylaxis with efanesoctocog alfa resulted in improvement in reduction of pain in patients with severe hemophilia A, when switching from prophylaxis with standard of care FVIII therapies to efanesoctocog alfa.
Disclosures
Wilson:Sanofi: Current Employment. Kragh:Sobi (Swedish Orphan Biovitrum AB ): Current Employment. Mshijid:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Dumont:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Willemze:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Santagostino:Sobi: Current Employment. Zhang:Sanofi: Current Employment. Kulkarni:Shire/Takeda: Other: supported clinical trials; BioMarin: Other: Supported clinical trials; Bayer: Other: Supported clinical trials; Sanofi/Bioverativ: Other: Supported clinical trials; Catalyst Biosciences: Membership on an entity's Board of Directors or advisory committees; Shire/Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Supported clinical trials; Pfizer: Membership on an entity's Board of Directors or advisory committees; Octapharma: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Other: supported clinical trials; Kedrion: Membership on an entity's Board of Directors or advisory committees; Genetech: Membership on an entity's Board of Directors or advisory committees; Bioverativ, BPL: Membership on an entity's Board of Directors or advisory committees; Unique: Other: supported clinical trials. von Drygalski:Bayer: Consultancy, Speakers Bureau; BioMarin: Consultancy, Speakers Bureau; CSL Behring: Consultancy, Speakers Bureau; Novo Nordisk: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Regeneron: Consultancy, Speakers Bureau; Sanofi Genzyme: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; uniQure: Consultancy, Speakers Bureau. Chowdary:Roche: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Chugai: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees; Freeline: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; Spark: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.